Buffers and QC
NanoCuvette™ One is used to quickly determine the quality of a buffer.
Download pdf here.
Buffer
A buffer is an aqueous solution composed of a mixture of a weak base and its conjugate acid, or a weak acid and its conjugate base. Because the pH of such solutions is very stable, buffer solutions are used to keep the pH constant in a wide array of applications, ranging from basic research to industrial production. In order to make the buffer compatible with physiological conditions, many buffer solutions also contain compounds that change the osmolality of the solution, such as salt (NaCl) or sugar (sucrose or glucose). Finally many buffers will contain additional ions with essential functions, such as improving the stability or activity of an enzyme. The correct composition of the buffer is often vital to the quality of the research or production at hand.
Quality control
To ensure correct pH and osmolality of the buffer a pH-meter and an osmometer can be applied. However, such measurements can be tedious, require the extra purchase of commercial calibration buffers, and will not necessarily reveal contaminations or small differences in essential ions. Instead, fast and easy quality control can be obtained by determining the refractive index (RI) of the buffer. The RI is a fundamental physical property that takes into account every compound present in the sample.
Principle
Conventionally, a specialized refractometer has been required for determination of the refractive index. However, with the innovative NanoCuvette™ One, a nanosensor is installed in a cuvette, allowing determination of the refractive index to be carried out in a standard spectrophotometer. Because most buffers do not absorb light in the UV-visible spectrum, traditionally, spectrophotometers have been unable to measure buffers.
Safety precautions
This method does not entail any safety precautions. Please refer to common laboratory practices.
Measurement
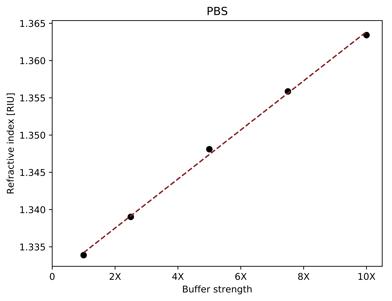
Figure 1. The sensor response, shown as refractive index, is linearly proportional to PBS concentration, which the free software will display.
- Materials and apparatus
The only apparatus required is a standard spectrophotometer and a computer with internet access. For each measurement a NanoCuvette™ One is required. - Sample preparation
A 10x stock of phosphate buffered saline (PBS) is prepared by dissolving 80 g NaCl, 2g KCl, 14.4 g Na2PO4 and 2.4 g KH2PO4 in 800 mL deionized (DI) water. When fully dissolved, the pH is adjusted to 7.4, and DI water is added to 1 L. Finally the PBS is serial diluted from 10x to 1x in DI water. - Measurement procedure
The spectrophotometer and computer are switched on and the free NanoCuvette™ One software is opened. For each sample a new NanoCuvetteTM One is used according to the software guidance. Following the last measurement the software will present the data as a variable of absorbance or refractive index, depending on the experimenter’s preferred choice of representation (Figure 1).

Figure 2. NanoCuvetteTM One measures refractive index and absorbance on the same sample, using only a standard spectrophotometer and the free NanoCuvetteTM One software.
Contact
www.nanocuvette.com